Abstract
Introduction - Selinexor is a first-in-class Selective Inhibitor of Nuclear Export (SINE) compound that binds and inactivates Exportin 1 (XPO1). Twice weekly (BIW) bortezomib in combination with dexamethasone (Vd) is an established therapy in relapsed and refractory multiple myeloma (RRMM). While the activity of bortezomib (bort) BIW in combination with other agents is efficacious, prolonged use is limited due to peripheral neuropathy (50-60%) as well as acquired resistance to bort. Strategies to identify new dosing regimes with high response rates, improved tolerability and the ability to overcome resistance are needed. Preclinical studies have shown that selinexor, when combined with bort, can restore sensitivity of bort-resistant MM, inhibiting tumor growth and increasing survival in murine MM xenografts. In this clinical trial (NCT02343042), we investigated the safety, tolerability and efficacy of the combination of selinexor, bortezomib and low dose dexamethasone (SVd) in patients (pts) with RRMM.
Methods - This phase 1b/2 dose escalation study was designed to determine the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) for SVd. The study enrolled pts with RRMM after ≥ 1 prior therapy. Pts with prior exposure or refractoriness to proteasome inhibitors (PI) were included, provided they were not refractory to bort as a last therapy. Selinexor was independently dosed escalated in once-weekly (QW, 80; 100 mg) or twice-weekly (BIW, 60; 80 mg) regimens. Bort (1.3 mg/m2 sc) was administered QW or BIW. Dexamethasone (dex) was given orally 40 mg QW or 20 mg BIW.
Results - As of July 15th, 2017, 42 pts were enrolled; 22 pts in the dose escalation and 20 pts in the expansion (RP2D) cohort. Median age was 64 years; 55% male, median of 3 (range, 1 - 11) prior lines of therapy. The MTD was not reached. Three pts in the BIW bort cohort were dose reduced to QW bort after Cycle 1. Common treatment related grade 1/2 adverse events (AEs) included: anorexia (57%), nausea (55%), fatigue (48%), and vomiting (26%). Grade 3/4 AEs included: thrombocytopenia (40%), neutropenia (19%) and anemia (12%). Importantly, peripheral neuropathy (PN) across all pts was limited to 6 patients (14%) (G1 4pts, G2 2 pts) of which 5 had prior bort exposure. Based on tolerability and efficacy, the RP2D of SVd is selinexor 100 mg, bort 1.3 mg/m2 and dex 40 mg, all QW (40% less bort and 25% less dex compared to the standard, approved BIW schedule of Vd). Among the 18 pts with non-PI refractory MM and ≤ 3 lines of prior therapy, the ORR was 83% and CBR 89%; the median follow up was 10 months and PFS was not reached. Ten of these pts remain on study. Among the 19 pts with PI (bort, ixazomib or carfilzomib) refractory MM, the ORR was 42% and CBR 68%. The median follow up was 9.2 months and the median PFS was 9 months. Six of these pts remain on study.
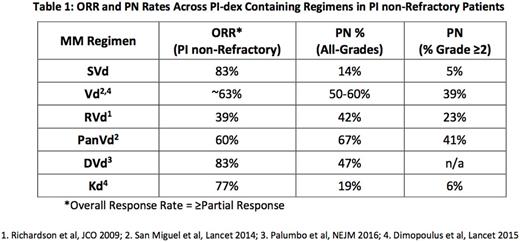
Conclusions - Selinexor in combination with weekly bort and dex is well tolerated and highly active in RRMM. The ORR of 83% and PN rate of 13% in PI non-refractory MM with 1-3 lines of prior therapy compares favorably to the previously reported ORR of 63% and >40% PN for Vd demonstrated in previous phase III trials. The high ORR with SVd is achieved with 40% less bort and 25% less dex and no overt major organ toxicities. Furthermore, in pts with PI refractory MM, the ORR of 42% and CBR of 68% support preclinical findings that selinexor re-sensitizes and overcomes resistance to PIs. SVd has shown durable responses regardless of prior PI sensitivity status and has an improved side effect profile. This data supports the ongoing phase 3 BOSTON study examining SVd vs Vd.
Bahlis: Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Sutherland: Janssen: Honoraria. Sebag: Celgene, Janssen: Consultancy. Lentzsch: Amgen: Consultancy; Caelum Biosciences: Other: leadership position and stock; BMS: Consultancy. Gasparetto: Janssen, BMS, Celgene, Takeda: Honoraria; Celgene: Research Funding; Janssen, BMS, Celgene: Other: Travel, accommodations, or other expenses paid or reimbursed; Janssen, BMS, Celgene: Consultancy. Bensinger: BMS: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Bayer: Research Funding; Sanofi: Consultancy, Research Funding; Acetylon: Research Funding. Kauffman: Karyopharm Therapeutics Inc: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Shacham: Karyopharm Therapeutics Inc: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Jeha: Karyopharm Therapeutics: Employment. Saint-Martin: Karyopharm Therapeutics: Employment. Shah: Karyopharm Therapeutics: Employment. Chen: Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Amgen: Honoraria; Abbvie: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.